HEPMA Update 3– November 2022
This month’s blog highlights a number of issues around how to use the system safely and most effectively.
Please contact nhsggc.hepma@ggc.scot.nhs.uk if you have any ideas for future blogs.
Business continuity
-
The business continuity laptop is an essential piece of equipment should HEPMA be unavailable for any significant period of time.
-
Does your ward (or clinical area) have a process to ensure checks happen daily?
-
Ensure someone is responsible for the checks on HEPMA.
-
Make sure you know where your kit is and where you can access the business continuity process (ward orange HEPMA folder).
-
Checklists for business continuity (BC) should be checked daily
-
Is the orange folder available to staff?
-
Does it record where your BC kit is?
-
Is the BC kit where it should be?
-
Is it plugged in to the mains?
- Is it plugged into the network point?
-
Is it turned on?
-
Does the login screen appear when opened?
-
If it is not working please report to nhsggc.hepma@ggc.scot.nhs.uk
or report to e-health via Service Now icon 
IF YOUR WARD IS MOVED OR CLOSES, DO NOT ATTEMPT TO MOVE BUSINESS CONTINUITY KIT YOURSELF. CONTACT E-HEALTH TO ENSURE IT IS MOVED CORRECTLY SO THAT IT CONTINUES TO FUNCTION.
Use of notes within HEPMA
-
The notes function within HEPMA allows us to record important information regarding the patient’s medication.
-
‘Note fatigue’ is a known phenomenon where users will ignore notes if there is too much information presented to them.
-
Please consider carefully if a HEPMA note is the correct way to convey the information you need to share.
-
Consider putting a suppression date on notes where possible or for drug notes suppressing when order discontinued (see screenshot, if viewing on mobile device switch to landscape)
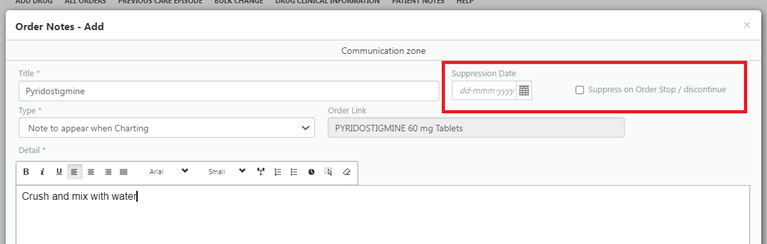
- Use the drug name as the title when creating a drug note to ensure people know what it relates to.
Changes to Paracetamol and Paracetamol containing products (e.g. co-codamol)
-
It is important to consider weight when prescribing paracetamol.
-
In order to ensure safe prescribing the HEPMA Clinical Reference Group has agreed to remove the default dose from paracetamol (and paracetamol containing products) outside its use in protocols. Doses in protocols will be discussed with the owners of those protocols before changes are made.
-
When prescribing paracetamol the user will need to add the dose of paracetamol (or paracetamol containing product) to be given.
Unlicensed Medicines and Conflict (Interaction/allergy) support
- Unlicensed medicines are highlighted in the HEPMA order screen (see screenshots below, if viewing on mobile device switch to landscape)
![]()
-
Unlicensed medications commonly do not have an entry in the conflict database, so will not show up as interacting with other medications and may not register if the patient is allergic to the constituents of the product.
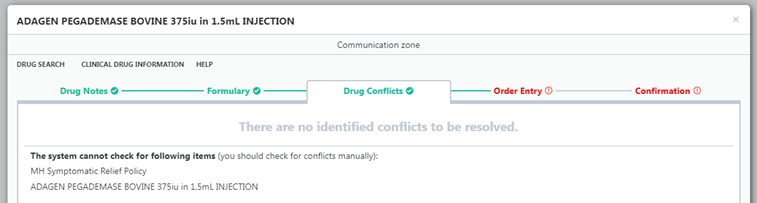
- Please take appropriate care when prescribing unlicensed medication and check with pharmacy if necessary for interactions.
Further information on HEPMA, including links to guides and training videos, can be found on StaffNet (GGC Network access required).
Previous Medicines Update blogs on HEPMA can be found by searching ‘HEPMA’ on GGC Medicines: Home.
Published: 02/12/2022. Medicines Update blogs are correct at the time of publishing
